Infecting Mosquitoes with Microfilaremic Blood
Materials
- Glass slides
- Glass cover slips
- Defibrinated Sheep's Blood (Hemostat Laboratories DSB100)
- Bleach
- Glass or electric blood feeders
- Parafilm
- Ring stand (glass feeders only)
Preparing blood for blood feeding
- All pipette tips, slides, serological pipettes, tubes, and other materials that come into contact with blood throughout this protocol must be treated with bleach prior to disposal. A bleach solution can be prepared in the bleach-specific pitcher in the insectary (labeled with yellow tape) by adding ~½ cup of bleach and 1 L of tap water.
Important: Remove the sucrose pad(s) at least 2 hr before feeding for LVP and AaSD mosquito species.
Starting with microfilaremic blood
-
Invert the tube of infected blood several times to mix the microfilariae (mf).
Note: If the microfilariae are shipped in multiple glass vials from the same subject, combine all of the vials into a single 50 mL conical tube and mix by inversion.
-
Add 20 µL of infected blood to 50 µL of dH2O on a glass slide and stir with the pipette tip to mix.
-
Add a cover slip to the slide. Adding the cover slip beginning at an angle and slowly lowering it will reduce bubbles in the middle of your slide.
-
Return the mf back to the incubator while counting.
-
Place the slide on a compound microscope and count the mf. Start at a corner of the cover slip and count the mf in the fluid outside of the coverslip. Once the perimeter has been counted, count the mf underneath the cover slip area using an s-pattern. (See figure below)
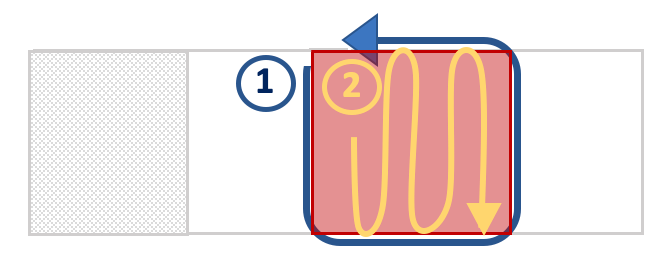
-
Repeat steps 2-5 three times. Take the average of the three mf counts and calculate the mf concentration in the microfilaremic blood stock.
-
In a 15 mL or 50 mL conical tube, dilute the microfilaremic blood with Defibrinated Sheep's Blood to the desired blood feeding concentration. Use C1V1=C2V2 to calculate how much microfilaremic blood and how much control blood to add to the conical tube.
Note: The blood feeders used for feeding hold 10 mL of blood. When calculating final volume use 11 mL for 1 mL overage. Multiply this by number of feeders you will need.
-
Appropriate concentrations for blood feeding:
-
B. pahangi: 100 - 120 mf / 20 µL
-
D. immitis: 90 - 120 mf / 20 µL
-
B. malayi:
-
-
Re-enumerate the mf in blood in duplicate to verify that it is within target concentration for the designated species (see target concentrations above). If concentration is off, re-calculate dilutions based on the new numbers and add defibrinated sheep's blood as needed. Additionally, verify mf have recovered (are moving) before feeding; otherwise they may be damaged or dead.
Starting with mf in IP fluid
-
Invert the tube in order to evenly distribute the mf throughout the IP fluid.
-
Add 20 µL of microfilaremic IP fluid to a glass slide and place a coverslip on top. Count the number of mf in the sample on a compound microscope using the pattern described above. This should be done in duplicate.
Note: It may be necessary to dilute the sample if the mf are too numerous to accurately count. A 1:10 dilution can be carried out by adding 20 µL of microfilaremic IP fluid to 180 µL RPMI-1640 medium, mixing, and dispensing 20 µL on a glass slide for quantification.
-
Determine how much volume of IP fluid you will need to get the desired mf concentration for blood feeding by using C1V1=C2V2. If a 1:10 dilution was used to count the microfilariae, multiply the concentration of mf/20 µL observed in step 2 by 10 to account for the dilution.
Note: The blood feeders used for feeding hold 10 mL of blood. When calculating final volume use 11 mL for 1 mL overage. Multiply this by number of feeders you will need.
-
Add the calculated amount of IP fluid to a 15 mL conical tube. Spin at 900 rpm for 10 min. You should see a pellet of mf at the bottom of the tube. Remove all supernatant without disturbing pellet. Add the final volume of blood you desire (11 mL x the number of feeders).
-
Re-enumerate the mf in blood in duplicate to verify that it is within target concentration for the designated species (see target concentrations above). If concentration is off, re-calculate dilutions based on the new numbers and add defibrinated sheep's blood as needed. Additionally, verify mf have recovered (are moving) before feeding; otherwise they may be damaged or dead.
Assembling glass blood feeders and feeding mosquitoes
-
Use the large glass blood feeders. Stretch a 2-square piece of parafilm pulled once in both directions and place it on the bottom of the glass feeder. Avoid wrinkles in the parafilm and secure the parafilm by smoothing it along the glass rim.

-
Cut a second piece of parafilm (2 squares wide, half square tall) and stretch it around the bottom edge of the glass feeder.
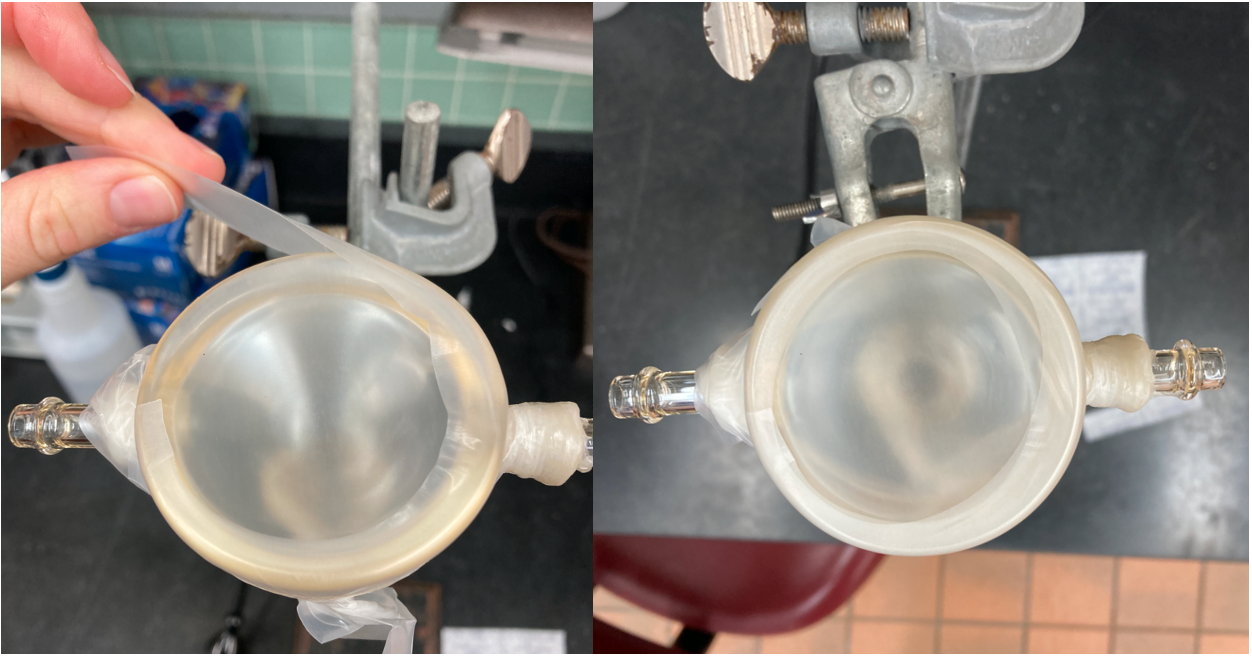
-
Secure the parafilmed glass feeder upside down on a metal ring stand and place on a cart in the insectary.

-
Setup the water circulator on a cart in the insectary by filling it with dH2O to the black fill line.
-
Attach the water circulator tubes to each inlet and outlet arm on the side of the glass feeders and wrap a piece of parafilm (2 squares wide, half square tall) around the connecting points. This will help control any water leaks.

-
Turn the water circulator on and set the temperature to 37°C.
-
In these feeders carefully place ~10 mL of diluted, infected blood into the top of the blood feeders using a disposable pipet. You need to make sure that the feeders are level so that the blood doesn’t pool in one side and unevenly distribute the mf.
-
Place a small strip of parafilm over the opening to the inner chamber where the infected blood was loaded into the glass feeder.
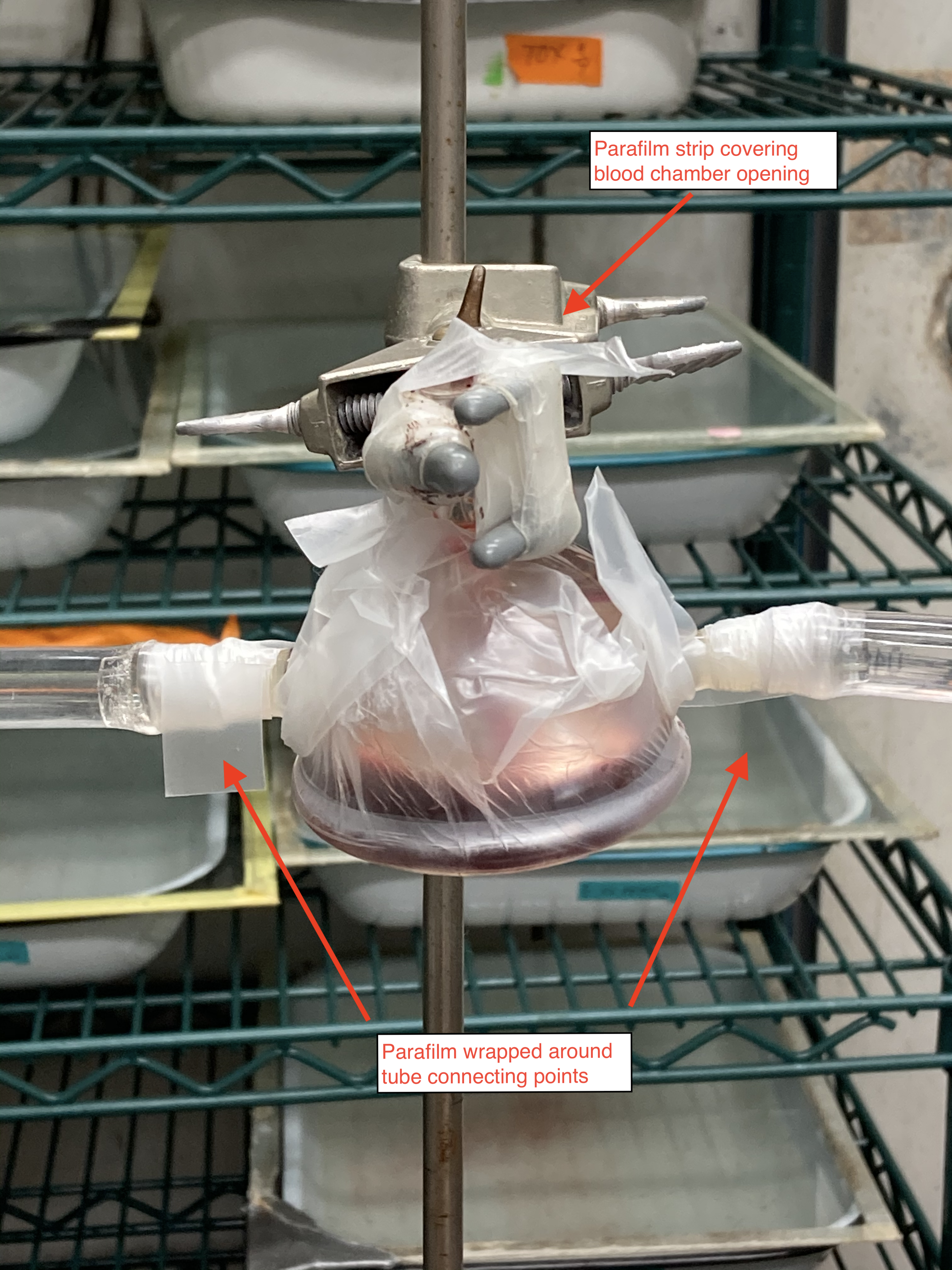
-
Let the water, glass feeders, and blood equilibrate to 37°C prior to feeding.
-
Place a carton of mosquitoes under a membrane feeder and slowly lower the feeder so that it rests atop the carton and is flush with the mesh of the carton.

-
Allow the carton of females to feed for 30 – 45 min. or until a majority of females have fed.
-
While the mosquitoes are feeding, print out a carton label according to the example below:

-
When feeding is complete, remove an aliquot of blood to confirm that the microfilariae are still alive and active. Do not separate blood fed from non-blood fed mosquitoes at conclusion of feeding.
-
Affix the carton label to the corresponding carton and place blood fed mosquitoes in the insect incubator.
Note: Mosquito cartons blood fed with B. pahangi or B. malayi must be double contained by placing the fed cartons inside the mesh cage located inside the incubator.
-
Allow the infection to incubate for 14-15 days before you extract L3 from the mosquitoes.
-
Other important notes:
-
Maintain the sucrose pads on the cartons daily. It will be important to communicate with the insectary manager or undergraduates to make sure sucrose pads are moistened every day including on the weekends.
-
If you notice an accumulation of dead mosquitoes in the bottom of the carton, suck out the dead mosquitoes, empty the suction device into a plastic container and freeze the container overnight. The mosquitoes can then be placed in the trash the next day after freezing.
-
Assembling electric blood feeders and feeding mosquitoes
-
Use the metal electric membrane feeder with the large screw hole. Stretch a 2-square piece of parafilm pulled once in both directions and place it on the indented side of the metal feeder. Avoid wrinkles in the parafilm and secure the parafilm by smoothing it along the rim and around the sides of the metal feeder.

-
Cut a second piece of parafilm (2 squares wide, half square tall) and stretch it around the edge of the metal electric feeder.

-
Cut off excess parafilm so the feeder will lay flat against the electric heating unit.
-
Fill the unit with microfilaremic blood until the bottom of the membrane is evenly covered. It will take 10-20 mLs of blood.

-
Once the feeder is filled with blood, place the plugs in the port holes and ensure they are flush with the metal feeder. If they are not flush, the feeder will not fit correctly on the electric unit and the blood will not be kept warm while feeding the mosquitoes.

-
Set up the electrical unit in the insectary and screw on the blood-filled membrane feeder. Turn the unit on and set the feeder on its side while the blood warms up for about 5 min.

-
Gently set the blood-filled feeder on top of the mosquito carton and allow the mosquitoes to feed for about an hour or until a majority of females have fed.

-
While the mosquitoes are feeding, print out a carton label according to the example below:

-
When feeding is complete, remove an aliquot of blood to confirm that the microfilariae are still alive and active. Do not separate blood fed from non-blood fed mosquitoes at conclusion of feeding.
Note: Mosquito cartons blood fed with B. pahangi or B. malayi must be double contained by placing the fed cartons inside the mesh cage located inside the incubator.
-
Allow the infection to incubate for 14-15 days before you extract L3 from the mosquitoes.
-
Other important notes:
-
Maintain the sucrose pads on the cartons daily. It will be important to communicate with the insectary manager or undergraduates to make sure sucrose pads are moistened every day including on the weekends.
-
If you notice an accumulation of dead mosquitoes in the bottom of the carton, suck out the dead mosquitoes, empty the suction device into a plastic container and freeze the container overnight. The mosquitoes can then be placed in the trash the next day after freezing.
-
Note about feeding SD Mosquitoes
These mosquitoes are fragile and the following precautions should be taken to achieve the best yield:
- SD should be panned out on a Friday and separated/counted on a Thursday
- Mosquitoes should not be blood fed on a Wednesday; if mf are arriving on a Wednesday because of a holiday, request they be sent a different week
- Only starve mosquitoes for four hours prior to blood feeding
- L3s should be extracted on day 13 after blood-feeding